Alternative materials for lithium-ion batteries Researchers from Braunschweig and Kyoto investigate ageing and stability of energy storage devices
The components of commercial lithium-ion batteries have been steadily improved over the past decades. Most recently, the progress has been smaller. The performance maximum with regard to the currently used materials seems to have been reached. The “OsabanPlus” project therefore focuses on alternative materials for lithium-ion systems. To this end, TU Braunschweig is cooperating with colleagues from Japan. We talked about this with Lukas Noll from the Institute of Energy and Process Systems Engineering.
In general, what are the characteristics of a high-performance battery?

Lukas Noll. Photo credits: Lukas Noll
The characteristics of a battery’s performance can be described simply in terms of three parameters: capacity (how long can I use my phone before I have to recharge it), power (how quickly can I then recharge my phone) and longevity or robustness (how often can I recharge the battery; when do I have to replace the battery). The “OsabanPlus” project focuses on a deeper understanding of the processes that affect or reduce longevity.
In which areas of application do certain battery characteristics play a special role?
Depending on the application of the batteries, the characteristics must be adapted. This is because some influence each other or are contrary to each other. It is not possible to optimise everything at once. For example, it is currently impossible to design a battery with a high capacity and good durability and at the same time expect a high charging or discharging performance. In addition, there are the issues of safety and the cost of a battery. This means that depending on the application of a battery, an individual compromise must be made between the performance, the safety and the costs.
Your project is about “energy density”, “cycle stability” and “reduced degradation of the electrolytes used”. What is meant by this and how does it interact?
The degradation or ageing of the components (electrolytes, electrodes) influence the cycle stability and thus the longevity or robustness of the system. The aim of the project is to investigate the ageing mechanisms in more detail. From this understanding, it should be possible to derive solutions that reduce the degradation of the cell components in order to increase the cycle stability and ultimately to profit reliably from the high energy density of the materials used.
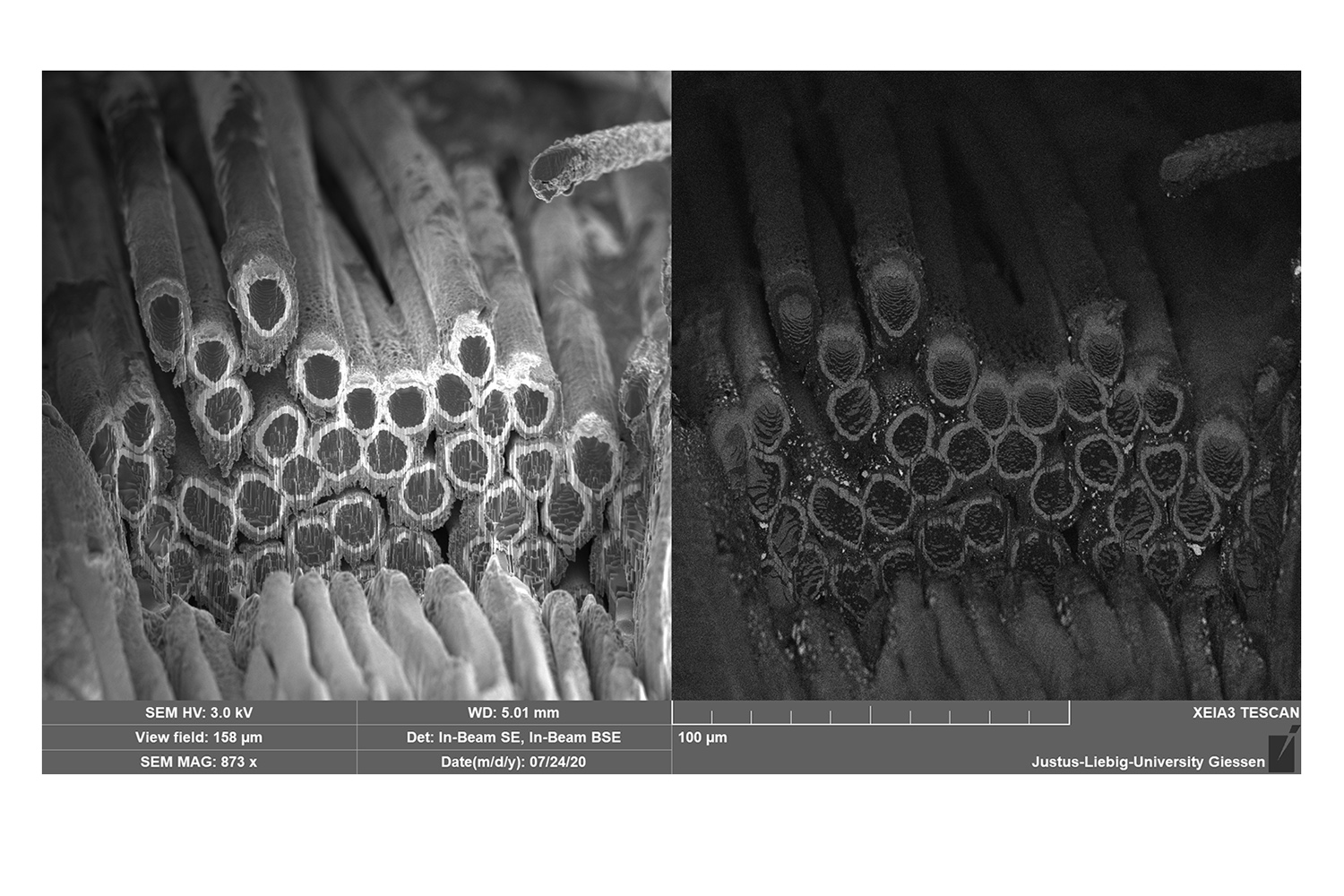
Magnified image with the scanning electron microscope of the structured 3D anode used in the project. The core of the electrode is made of carbon bundles (black; approx. 10 µm in diameter), each of which is coated with zinc oxide (white; approx. 1 µm thick). This is where the lithium ions from the electrolyte are stored. Picture credits: Julian J. A. Kreißl , B.Mogwitz. Reprinted with permission from ACS Appl. Mater. Interfaces 2021, 13, 30, 35625-35638. Copyright 2022 American Chemical Society.
You deal with “conversion anodes”. What is that and what should they be able to do better?
The current status quo of the negative electrode (the anode) represents graphite, which stores lithium ions via an “intercalation mechanism”, i.e. a storage and retrieval of lithium ions. Compared to graphite, conversion anodes, for example made of zinc oxide, can store more lithium ions via a modified storage mechanism. This leads to a higher specific energy density. In principle, the successful implementation of such conversion materials could lead to a considerable increase in energy density and, at the same time, a reduction in the cost per kilogram or litre of lithium-ion batteries. At present, however, it is proving difficult to realise long-lasting batteries with these electrodes.
What is the reason for this?
The reason is continuous side reactions of the electrolyte with the electrode material as well as a volume change of the active materials of more than 100 per cent during the storage/retrieval of lithium ions. This process is called “breathing” of the electrode. Breathing can lead to cracking and detachment of the active materials during charging and discharging of the battery. These problems significantly limit the use of the conversion materials mentioned.

Pictorial representation of the investigation of structured electrode materials. Photo credits: Julian J.A. Kreißl and Daniel Schröder
How could we get breathing or volume change under control?
We are testing 3D-structured anodes for this purpose. The three-dimensional structure of the electrode means that the active material, which enables the redox reaction and thus the energy storage in a battery, has been applied to a three-dimensional current collector, such as a porous carbon. By embedding the active material in very small pores, the active surface area is increased. This can increase the performance of the electrode. The pores buffer the enormous volume expansion during operation and protect the active material from contact loss. This ultimately increases the longevity of the electrode.
You are a junior scientist. What will be your topic in the project? What is the appeal of battery research for you?
The focus of my work at the institute will be to set up and put into operation a measuring set-up for measuring the acoustic emissions of batteries. This will then be used to investigate, among other things, the conversion electrodes mentioned and their change in volume during operation.
I came to Braunschweig for my Master’s degree to focus my studies on electrochemical systems such as lithium-ion batteries, because in my eyes they play an important role towards a sustainable future. Since a lot is happening in this field at the moment and there is still a lot of research to be done, working at a research institution is all the more exciting!
And what does the cooperation with Japan look like?
The cooperation with the project partner from Japan runs through a joint call of the BMBF to strengthen scientific cooperation in the field of battery research. In this project, we receive materials from the project partners in order to then examine them. Thus, it is about the exchange of samples as well as knowledge. In addition, depending on the current situation, the project also allows for a project meeting and a research stay with activities in the laboratory of the project partner at the Katsura Campus in the laboratory of Professor Takeshi Abe of Kyoto University in Japan.
The project’s logo is a real eye-catcher. How is the illustration to be understood?
Logos and mascots are an important part of the culture in Japan and can be found on almost every street corner in everyday life. Therefore, it was important to us to design a suitable logo for this German-Japanese project that would be convincing in terms of visual language. First of all, the crane is shown as a symbol for Kyoto, as these animals can be found in large numbers along the Kamogawa River near the city centre. A ray runs out of the crane onto a “pond” of structured anode elements. The beam symbolises the analytical methods used in the project to examine the 3D-structured battery electrodes. All of this is against the background of the setting Japanese sun and a battery cell.
Thank you.