Picture of the month: Microstructure of the stomach wall – The key to healthy digestion From the Institute for Mechanics and Adaptronics
Whether heart valves, urinary bladders or tracheas: through tissue construction and tissue engineering, these organs have already been successfully replaced artificially in patients. Other organs, such as the stomach, are much more complex. They consist of different tissues, different cell types and are also permeated by countless blood vessels. In order to improve the quality of life of gastric resection patients with a new stomach replacement therapy, the biomechanics laboratory is researching the mechanical properties of a healthy stomach on different size scales.
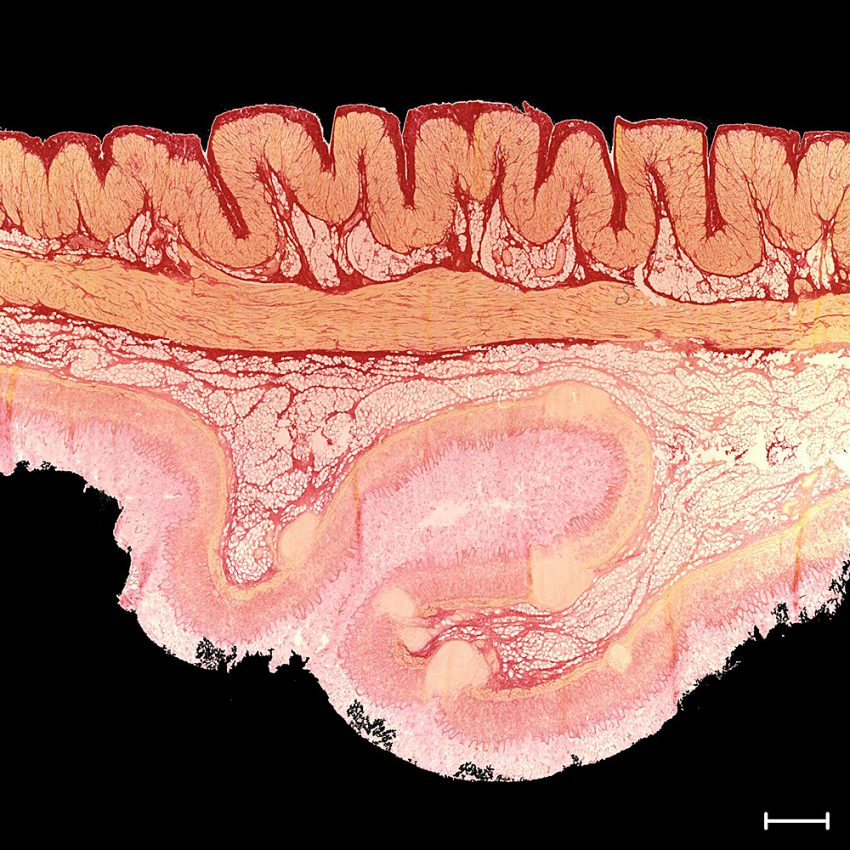
Histological section through the stomach wall. The different layers can be seen. Smooth muscle cells are stained yellow, collagen red and fat is stained white. Bar: 1 mm. Photo credit: Institute for Mechanics und Adaptronics/TU Braunschweig
Understanding mechanical properties
Gastric resection, the partial or total surgical removal of the stomach, has become a reliable procedure to achieve complete removal of the tumour lesion. Despite its curative potential, complications remain a common clinical problem associated with a significant reduction in quality of life. Various gastric replacement techniques have been developed to improve quality of life, although a major criticism is the subsequent limited capacity for food intake. An alternative may be a stomach or partial stomach created by tissue engineering. Tissue engineering has enormous potential for regenerative medicine.
For the successful realisation of such an idea, ideal materials should have biological compatibility and mechanical reliability in terms of tissue engineering. For this, it is necessary to fully understand the mechanical properties of the healthy stomach at different size scales.
In a research project, the Institute for Mechanics and Adaptronics is working in its biomechanics laboratory on the experimental, multiscale characterisation of the stomach, i.e. from the individual cell to the stomach tissue to the entire stomach. The mechanical behaviour of engineering materials is influenced by their heterogeneous microstructure. Due to large length scale differences, it is usually not possible to explicitly capture this influence in component simulations. Therefore, the Institute’s biomechanics laboratory relies on multiscale approaches in material modelling. The heterogeneous microstructure is made explicit on a small length scale and its influence on the macroscopic material behaviour is determined in averaged form with the help of numerical homogenisation. Not only the passive properties on the different length scales are determined, but also the active properties necessary to transport and comminute the gastric pulp within the stomach.
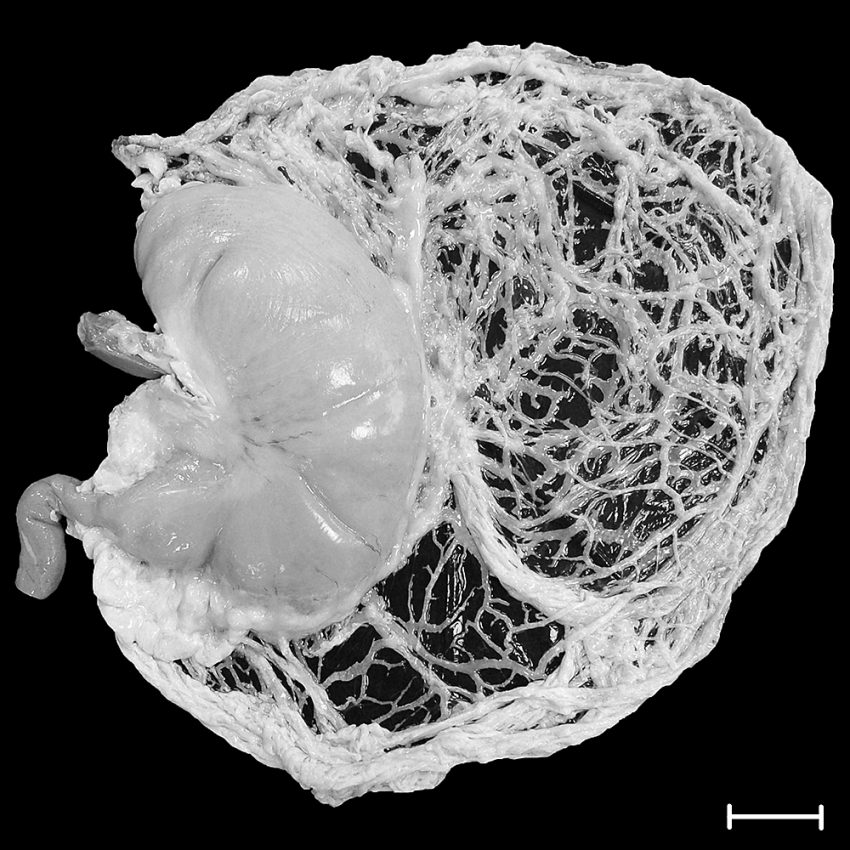
A stomach filled with saline solution. The bands by which the stomach is suspended in the body are clearly visible. Bar: 5 cm. Photo credit: Institute for Mechanics und Adaptronics/TU Braunschweig
The stomach, as the most important part of the gastrointestinal tract, is a J-shaped contractile organ located between the oesophagus and the duodenum and anchored to ligaments in the body.
The highly structured microstructure of the stomach wall is essential for the three skin functions of the stomach – storing, crushing and transporting the food pulp. Using immunohistological techniques, the microstructure of the stomach wall was made optically visible at the Institute for Mechanics and Adaptronics.
Microstructure of the stomach wall
In the histological section through the stomach wall, the muscle cells are coloured yellow, collagen red and fat structures white. It is easy to see that the stomach wall has different layers that enable the complex mechanical properties, both active and passive. As the study shows, the microstructure controls the macromechanical behaviour of the stomach wall. The project also shows that the microstructure of the stomach wall depends on the stomach wall position. Thus, the stomach is anatomically divided into fundus, corpus and antrum. Here, too, the comparison shows clearly different microstructures, which explains, among other things, the complex gastric wall contractions.
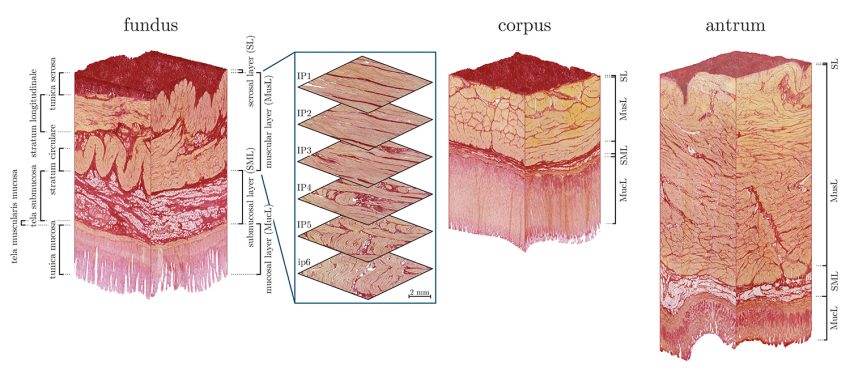
Photorealistic three-dimensional reconstruction of the stomach wall in the fundus, corpus and antrum based on histological sections. Photo credit: Institute for Mechanics und Adaptronics/TU Braunschweig
The results of these investigations were recently published in the internationally renowned journal Acta Biomaterialia1. These data are further used to develop multiscale multi-field models that can describe the contraction behaviour of the stomach very impressively. A first numerical approach was also published in Acta Biomaterialia2. In the future, the model will serve researchers in predicting gastric contractions, – including those of diseased stomachs -, as highlighted by a recent book article3.
