St. John’s wort: TU Braunschweig discovers important steps in biosynthesis
St. John’s wort is one of the best known medicinal plants. There are more than 500 species worldwide. Its complex constituents have promising medicinal properties. However, they are difficult to isolate and synthesise. Scientists at the Institute of Pharmaceutical Biology at Technische Universität Braunschweig have identified two novel enzymes that form alternative molecular variants. This opens up the possibility of obtaining the complex ingredients using biotechnology. The results were published in the renowned journal “Nature Communications”.
St. John’s wort extracts are used to treat mild to moderate depression. Its effectiveness has been demonstrated in numerous clinical trials. The main active ingredient is hyperforin. Over 1000 similar compounds have now been identified in other St. John’s wort plants. What they all have in common is a complex chemical structure. Total synthesis, i.e. the complete chemical reconstruction of natural substances from basic substances, is therefore difficult.
Isolating the active compounds is also a challenge, as the levels in the plants are low. On the other hand, the compounds have many interesting pharmacological effects, such as anti-tumour (anti-cancer) and antibacterial activities. In order to develop an alternative source for their extraction, the Institute of Pharmaceutical Biology is trying to transfer the biosynthesis of St. John’s wort to microorganisms. Microorganisms are relatively easy to grow in bioreactors as cell factories. The Center of Pharmaceutical Engineering (PVZ) at TU Braunschweig is predestined for this process.
However, in order to reconstruct plant biosynthesis in microorganisms, it is necessary to know the steps of the biosynthetic pathway. This is where a breakthrough has now been made. The constituents of St. John’s wort consist largely of two molecular variants derived from the same precursor. A St. John’s wort (Hypericum sampsonii) that is common in China is particularly rich in complex constituents and contains both molecular variants. In 2010, Dr. Benye Liu brought this plant to TU Braunschweig. Two new bifunctional enzymes have now been identified in this plant. They enlarge the precursor by a residue of five carbon atoms and also manage two positionally different ring bridges. This results in the two alternative bridged molecule variants, which are decorated with side chains. These enzymatic products and the complex constituents were analysed in close collaboration with the Max Planck Institute for Chemical Ecology in Jena.
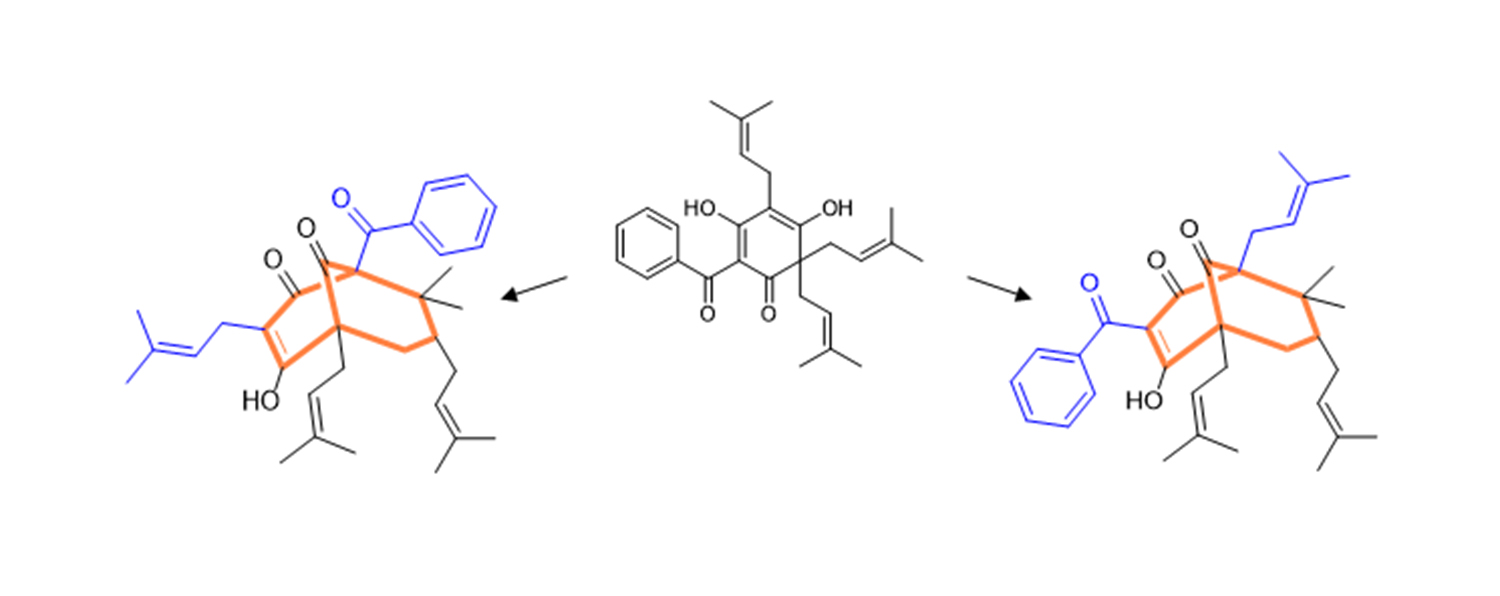
Molecular variants: The same precursor (centre) is converted into different molecular variants by two enzymes. Their core (red) is typical for many complex ingredients of St. John’s wort. Photo credit: Ludger Beerhues/TU Braunschweig
Another collaborator was an institute at the Chinese Academy of Sciences in Tianjin. Computer simulations were used to create models for both enzymes to explain their regiodivergent prenylative cyclisations – biochemical reactions in which a hydrocarbon (isoprene) is added to an open-chain side residue of the starting molecule, leading to the formation of a bicyclic (two-ring) product molecule. These reactions are important in the biosynthesis of complex organic compounds and are an inspiration for natural product chemistry and the biotechnological production of bioactive molecules.
Interestingly, the common precursor is bound upright in the first enzyme and vice versa in the second. The amino acids essential for stabilising these binding positions were identified and their mutual exchange transferred one enzyme into the other.
The bridged products of the enzymes studied here are likely to be the precursors of even more complex, cage-like compounds that also occur in St. John’s wort and are of pharmaceutical interest. These findings should facilitate the search for the downstream enzymes that build these more complex compounds. This will increase the chances of creating suitable microbial production platforms to make the fascinating compounds of St. John’s wort available for preclinical development.
Original publication:
Lukas Ernst, Hui Lyu, Pi Liu, Christian Paetz, Hesham M.B. Sayed, Tomke Meents, Hongwu Ma, Ludger Beerhues, Islam El-Awaad & Benye Liu (2024) Regiodivergent biosynthesis of bridged bicyclononanes. Nature Communications 15, 4525 (2024). https://www.nature.com/articles/s41467-024-48879-w
